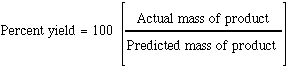Objectives
- to observe the reaction between solutions of sodium carbonate and calcium chloride
- to determine which of the reactant is the limiting reactant and which is the excess reactant
- to determine the theoretical mass if precipitate that should form
- to compare the actual mass with the theoretical mass of precipitate and calculate the percent yield
After we put two kinds 25ml of solution: Na2CO3 and CaCl2, the precipitate will form.


Set up the filtering apparatus like the picture show above. And pure the mixture slowly and carefully into the filter funnel. It takes some time to complete the filtering process.Then put the filter paper away and dry it.
After the filter paper is completely dry, we can weigh the mass of filter paper to calculate the mass of precipitate.

Accoding to the molarity of the solution we used for this lab, we can calculate the mass of Na2CO3 and CaCl2. According to chemical equation, we can determine which one is the limiting reactant and which one is excess reactant.
Then we can calculate the mass of CaCO2 we expect to get.
Use the formula % yield= mass actual produced/mass expect produced*100%, we can calculate the % yield of this lab.