 Reactions rarely produce the
predicted amount of product from the masses of reactants in the reaction .
Reactions rarely produce the
predicted amount of product from the masses of reactants in the reaction .The percent yield is defined as
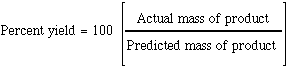
The predicted yield is
determined by the masses used in a reaction and the mole ratios in the balanced
equation. This predicted yield is the "ideal". It is not always possible to get
this amount of product.
1. Balancing The Chemical
Equation:
The first step in finding theoretical and percentage yield is
to balance the relevant chemical equation.
2. Finding The Limiting
Reagent:
- this is the reactant which the product yield depends on, as it is not in excess.
- To determine which reactant is the limiting reagent
1(b). Multiply the amount used (in mL) by its density, then divide by its molar mass
2. Multiply the mass (your answer from steps 1(a) or 1(b)) by the number of moles of the reactant used in the reaction.
3. Percent Yield
The percentage yield is the
ratio between the actual yield and the theoretical yield multiplied by 100%. It
indicates the percent of theoretical yield that was obtained from the final
product in an experiment.

When we make something in a chemical reaction, and separate it from the final mixture, it will still have small amounts of other substances mixed with it. It will impure.
The formula for percent purity is:



Finding Percent Purity
When we make something in a chemical reaction, and separate it from the final mixture, it will still have small amounts of other substances mixed with it. It will impure.
The formula for percent purity is:

Example:
The aspirin from the above experiment was not pure. 121.2 g of solid was obtained, but analysis showed that only 109.2g of it was aspirin. Calculate the percent purity of the product.
Solution:
Percent purity = 109.2 ÷ 121.2 × 100% = 90.0%

No comments:
Post a Comment