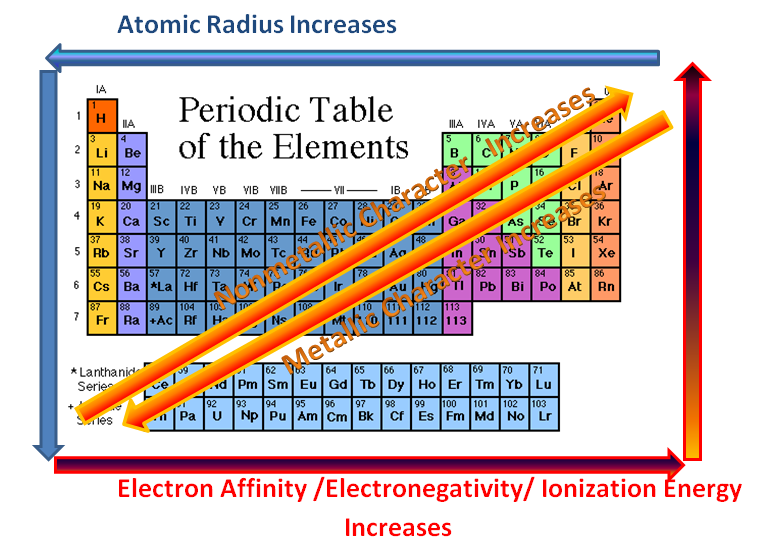
Alkane
- An alkane is a hydrocarbon in which all the carbon atoms are connected by single bond.
- Number the carbon atoms in the parent chain.
- Using the correct substituent name (methyl, ethyl, chloro,etc.), and the carbon atom number on the parent chain.
- If a particular substituent occurs more than once, use a prefix (di-, tri-, tetra-) to indicate the number of those substituents.
- List the alkyl substituents in alphabetical order.
Formula | Name |
CH4 | Methane |
C2H6 | Ethane |
C3H8 | Propane |
C4H10 | Butane |
C5H12 | Pentane |
C6H14 | Hexane |
C7H16 | Heptane |
C8H18 | Octane |
C9H20 | Nonane |
C10H22 | Decan |

Cycloalkanes
- Hydrocarbon chains which connect in a head-to-tail circle are called cyclic hydrocarbons or cycloalkanes.
Rules
- A single substituent does not use a number to indicate the position of attachment
- If there are more than on substituent, the first substituent is assumed to be at the "1" position and the remaining substituents are numbered either clockwise or counterclockwise so as to have the lowest set of overall values.
Alkyl Halides
Rules
- Attached F, Cl, Br and I atoms are called "fluoro", "chloro", "bromo" and "Iodo" groups
- If more than one of the same kind of galogen is present, use the prefix di, tri, etc.
- If a compound contains both alkyl and halo groups, list the attached groups in alphabetical order.
Mutiple Bonds
- An alkene is an organic compound containing a carbon-carbon double bond.
- An alkyne is an organic compound containing a carbon-carbon triple bond.
Rules
- If a double bond is present, change the "ane" ending of the parent hydrocarbon to "ene"
- If a triple bond is present, change the "ane" ending of the parent hydrocarbon to "yne"
- Use a number to indicate the lower numbered carbon involved in the bond. The number goes immdiately in front of the name of the parent hydrocarbon.
- Number the parent hydrocarbon to give the double/triple bond the lowest possible mumber.
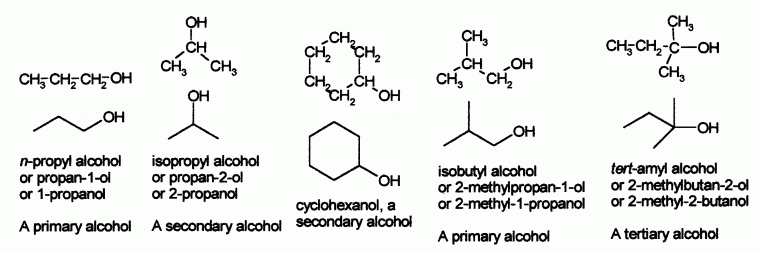
- An alcohol is an organic compound containing an OH group.
- Number the hydrocarbon chain to give the lowest possible number to the OH group.
- Place the number immediately before the name f the parent hydrocarbon, separated by a dash. Alkyl groups are placed in front of the number for the OH.
- Indicate the presence of an OH group by changing the "e" ending of the hydrocarbon chain to "ol".
- An Aldehydes is an organic compound containing a C=O group at the end of a hydrocarbon chain.
Rules:
- Number the-longest carbon chain starting with the -CHO group.
- Name the parent compound by using the alkane name and replacing the -e ending with an -al ending.

- A ketons is an organic compund containing a C=O group at a psoition other than at the and of a hydrocarbon.
- Number the-longest carbon chain starting so that the –C=O group is attached to the carbon atom with the lowest number.
- Name the parent compound by using the alkane name and replacing the -e ending with an -one ending.

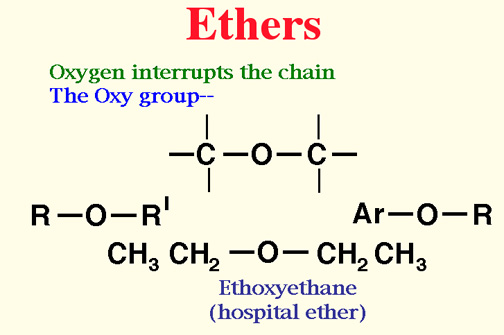
Ethers
- An ethers is a organice compound in which an oxygen joins two hydrocarbon groups.
- Start with the side group, add "oxy" to the side group name.
- An amine is an organic compound containing an NH2 group.
- Amines are organice base and react with acids.
- Identify the names of the alkyl groups bonded to the nitrogen atom
- Simply replace the alkane -e ending with -amine.
- The format is as follows: (alkyl name)(-amine)

Amides
- An amides is an organic compound containing a CONH2 group.
- Amides are commonly named similar to a carboxylic acid, replacing the –oic acid suffix with amide.

- An carboxylic acid is an organic compound whcih contains COOH group.
- Number the-longest carbon chain starting with the -COOH group. Name the parent compound by using the alkane name and replacing the -e ending with an –oic acid ending.