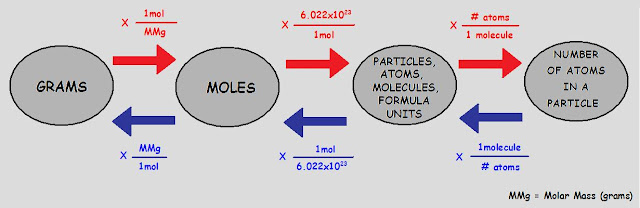
Separation
Component in a mixture retain their identities.
The more similar the properties are the more differernt it is so to seperate them.
Some Basic Techinques
Filtration
Floatration
Distillation
Chromatography
Crystallization and Extraction
Hand Separation and Evaporation
Hands separation (solids and solids)
A mechanical mixture can be separated by using a magnet or sieve.
Evaporation
Boil away the liquid and the solid remain.
Filtration
Pass a mixture through a porous life.
Use filter paper --residue left in filter paper,filtrate goes through filter paper.
Crystallizaton
Precipitation
Solid are then separated by filteration or floatation.
Use a saturated solution of a desired solid.
Evaporate or cool-solid comes out as pure cystals, then filtered.
Gravity Separation (solid base on density)
A centrifuge whirls the test tube around at high speed forcing the denser materials to the botton.(works best for small volume)
Solvent extraction
Mechenical Mixture:Use liquid to dissolve and solid but not the other,so the desied solid is left behind or dissolved.
Solution--solvent is insoluble with solvent already present.
Distillation (liquid in liquid solution)
Heating a mixture can cause low boiling component to volatilize(vaporize).
Then collect and condense the volatilied component.
Chromatography
Flow the mixture over a material that retain some components more than others.so different component flow over the material at different speeds
A mobile phase sweeps the sample over a stationary phase (as the wind sweeps the swam over the flower bed)
Sheet chromatography Paper chromatography (PC)
Stationary phase is liquid soaked into a sheet or strip of paper
Components appear as separate spots spreeds out on the paper after drying or "developing"
Sheet Chromatography Thin layer Chromatography
Stationary phase in a thin layer of absorbent containg a sheet of plastic or glass.
Some components bond to the absorbent strangly other more wearly.
Heating Curve
A: Solid; closely packed in an orderly manner, strong bonds and vibrates at a fixed position
A----B : heat energy converts to kinetic energy (KE), vibrate a little faster, temperature increase
B: Same to "A"
B----C: temperature remains the constant, because heat is used to overcome forces of attraction that hold particles together.
_Melting point_ (solid --- liquid):heat absorbed is heat of fusion
C: Liquid;all solid has melted
C----D: Temperature and KE increases, particles move faster
D: start turning from liquid to gas
D----E: temperature stays the same since heat is used to overcome the forces of attraction that hold the particles together
_Boiling point_ (liquid -> gas)
E: Gas
E----F: Heating continues, KE and temp increases; particles move faster
Cooling Curve
P: Gas; particles have more high energy and moves quickly
P→Q: KE decreases, particles are getting closer together, temperature decrease
Q: start to form intermolecularbonds, condensation begins, liquid starts to form
Q→R: condensation, stronger bonds formed,temperature remains constant, heat energy released called latent heat of vaporization
_Boiling point_ (gas --- liquid)
R: Liquid state
R→S: Temperature and KE decreases, molecules lose energy, vibrate slower and moves closer to each other
S: Starts to freeze into solid
S→T:particles arrange in an ordered manner
_Freezing point_ (liquid to solid)
T: Solid
T→U: Temperature decreases
U: Substances has reached room temperature
U→V: Remains as a solid in room temperature