
EXAMPLE:
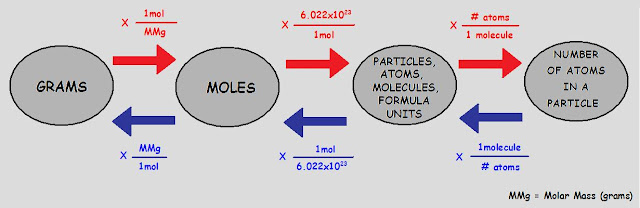
1.Determine the number of moles for 1 molecule of (NH4)2SO4.
Molar mass of (NH4)2SO4 is 132.1g.
1mol(NH4)2SO4 x 1mol/6.022x10^23mol(NH4)2SO4 x132.1g/1mol
=2 x (10)^-22
2.Determine the number of grams for 1.5x10^15 molecules of CuCl2.
Molar mass of CuCl2 is 134.5g
1.5x10^15mol CuCl2x1moe/6.022x10^23mol CuCl2=2.4x10^-9
3.How many moles are there in 92.0g of Lead?
92.0gx1mol/207.2g=0.44mol

No comments:
Post a Comment